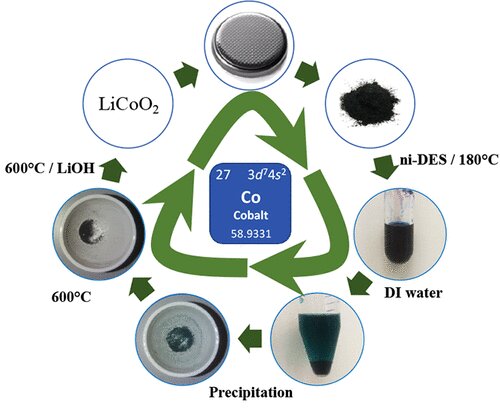
Scientists Find Cobalt Can Be Harvested from Urine: In a stunning new breakthrough, researchers have discovered that cobalt—one of the most critical metals used in electric vehicle (EV) batteries—can be recovered from human urine. Yep, that’s right: the same waste we flush away every day might just hold the key to a greener, more sustainable tech future.
This revolutionary technique, developed by teams at Linnaeus University in Sweden and the Indian Institute of Technology Madras (IIT Madras), utilizes a safe and natural mixture of urine and vinegar (acetic acid) to extract cobalt from used lithium-ion batteries. It’s an eco-friendly solution to one of the dirtiest problems in modern technology: battery waste and unsustainable mining.
Scientists Find Cobalt Can Be Harvested from Urine
This wild but wonderful idea—that we can harvest cobalt from urine—could flip the script on how we deal with e-waste, critical mineral shortages, and environmental damage. It’s a low-cost, low-risk, high-reward solution that reimagines waste as a resource.
If scaled globally, it could:
- Reduce mining pressure on vulnerable regions,
- Cut down battery production emissions,
- Drive a more circular, ethical, and affordable clean energy economy.
This isn’t just about chemistry. It’s about vision. And that vision starts with a single flush.
| Highlight | Details |
|---|---|
| Recovery Rate | Up to 97% of cobalt successfully extracted |
| Energy Use | Process operates at 180°C (vs 1400°C in traditional methods) |
| Chemical Safety | Uses non-toxic solvents (urea + acetamide) |
| Sustainability Fit | Reduces carbon emissions by ~65% compared to smelting |
| Research Institutions | Linnaeus University (Sweden) and IIT Madras (India) |
| Industry Applications | Battery recycling, EV manufacturing, circular economy programs |
| Regulatory Compatibility | Supports U.S. Inflation Reduction Act and global clean tech goals |
| Status | Validated in labs, now progressing toward pilot-scale testing |
Why It Matters: A Global Problem, A Local Solution
Cobalt is essential for stabilizing energy density in lithium-ion batteries, used in everything from smartphones to Tesla cars. But it comes at a cost. Over 70% of the world’s cobalt supply comes from the Democratic Republic of the Congo, where mining operations have been linked to environmental degradation, unsafe working conditions, and even child labor.
By 2030, global demand for cobalt is expected to triple, driven by the booming EV market and the global transition to renewable energy. This raises a tough question: how do we meet this demand without further damaging the environment or violating human rights?
The answer may be sitting in your toilet tank.
Breaking Down the Process: How Does It Work?
At first, it might sound like science fiction—but the chemistry checks out. Here’s a simplified breakdown of how this method works.
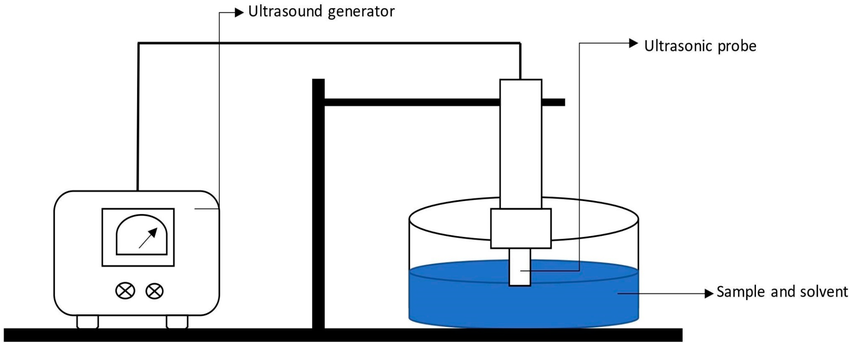
Step 1: Battery Waste Collection
Old lithium-ion batteries are shredded into what’s called “black mass“—a mixture of various metals including cobalt, nickel, manganese, and lithium. This powdery waste is often considered toxic and is hard to separate using traditional methods.
Step 2: Prepare the Solvent
The scientists created a Deep Eutectic Solvent (DES)—a green solvent—by combining urea (found in urine) and acetamide (related to vinegar). DESs are known for being biodegradable, non-toxic, and affordable.
Step 3: Cobalt Extraction
The black mass is mixed into the DES and gently heated to 180°C. At this temperature, the solvent dissolves the cobalt selectively, separating it from other materials like plastics or aluminum.
Step 4: Metal Recovery
Once the cobalt is dissolved into the solution, it can be recovered as cobalt salts through simple chemical precipitation. These salts can then be refined and reused in manufacturing new lithium-ion batteries.
Step 5: Solvent Recycling
The solvent can be reused multiple times, reducing waste and operational costs. Any leftover residues are safer to handle than those from traditional chemical processes.
Why Is This Better Than Traditional Recycling?
Let’s compare the new urine-based process with conventional recycling methods:
| Method | Temperature | Chemicals Used | Cobalt Recovery | Environmental Impact | Estimated Cost |
|---|---|---|---|---|---|
| Pyrometallurgy | ~1400°C | Strong acids, oxidants | ~60% | High emissions | $12–18/kg |
| Hydrometallurgy | ~300°C | Sulfuric/Nitric acid | ~70% | Moderate toxicity | $8–12/kg |
| Urine-Based Method | 180°C | Urea + Acetamide | Up to 97% | Low (non-toxic waste) | $5–8/kg |
The urine method:
- Uses far less energy (saves up to 85% on fuel costs),
- Requires no harmful chemicals,
- Produces minimal toxic waste, and
- Is much cheaper on a per-kilogram basis.
The Environmental Impact: Greener Than You Think
Battery recycling is one of the most pressing challenges in sustainable tech. Traditional methods generate tons of CO₂, release toxic fumes, and leave behind hazardous sludge. In contrast, this urine-based method:
- Cuts CO₂ emissions by an estimated 65% per ton of cobalt,
- Reduces hazardous waste volume by over 80%, and
- Has the potential to create zero-liquid-discharge systems for waste streams.
Moreover, using human waste as a chemical input opens the door to integrated waste-to-resource systems, where municipal wastewater can be transformed into a raw material source for clean tech manufacturing.
Expert Voices
Professor Ian Nicholls of Linnaeus University said, “Our process shows that valuable metals can be recovered in an environmentally friendly way. We’ve moved away from dangerous acids and found a sustainable, repeatable method that can work at scale.”
Meanwhile, Dr. Ramesh Govindaraj at IIT Madras added, “This is not just lab curiosity. We’re designing pilot-scale setups to test how well this works under industrial conditions. The implications for India and other tech-producing countries are huge.”
Scientists Find Cobalt Can Be Harvested from Urine: Who Can Benefit?
1. Battery Manufacturers
Companies like Panasonic, LG, and CATL can embed this method into their battery production supply chains, reclaiming materials and reducing reliance on unstable cobalt sources.
2. EV Producers
Brands such as Tesla, Ford, and Rivian can close the loop by recycling battery packs at end-of-life using this green method.
3. E-Waste Facilities
Recycling plants in the U.S., India, and the EU can implement this process to comply with stricter waste disposal laws while improving efficiency.
4. Public Utilities
Municipalities can explore how their sewage systems might one day feed into clean material supply chains.
Real-World Example: A U.S. Recycling Plant
Imagine a facility in Ohio currently recycling EV batteries using standard smelting methods. They’re burning fossil fuels, handling acids, and generating toxic byproducts. With the new method:
- Operating temperatures drop from 1400°C to 180°C
- Toxic fumes are eliminated
- Cobalt recovery increases by 30–40%
- Material costs drop by up to 50%
The result? Cleaner air, lower bills, and more sustainable production.
How This Aligns With U.S. Policies?
This breakthrough dovetails neatly with several major U.S. policy initiatives:
- The Inflation Reduction Act (IRA) offers tax credits and funding for domestic battery recycling projects.
- The DOE’s Battery Recycling Prize encourages scalable, low-cost solutions for lithium-ion battery materials.
- The EPA’s Green Chemistry Program promotes exactly this type of eco-innovation.
Companies that adopt this method may qualify for federal funding, innovation grants, and sustainability certifications.
No Gas, No Diesel; Rehlko’s New Engine Shocks America with Bold Electric-Hydrogen Fusion
It’s Official: Honda’s First Electric Motorcycle Is Here; Meet the Wuyang‑Built E‑VO
Not Gold, Not Copper—Egyptians Used Iron From Space in Sacred Objects, New Study Reveals